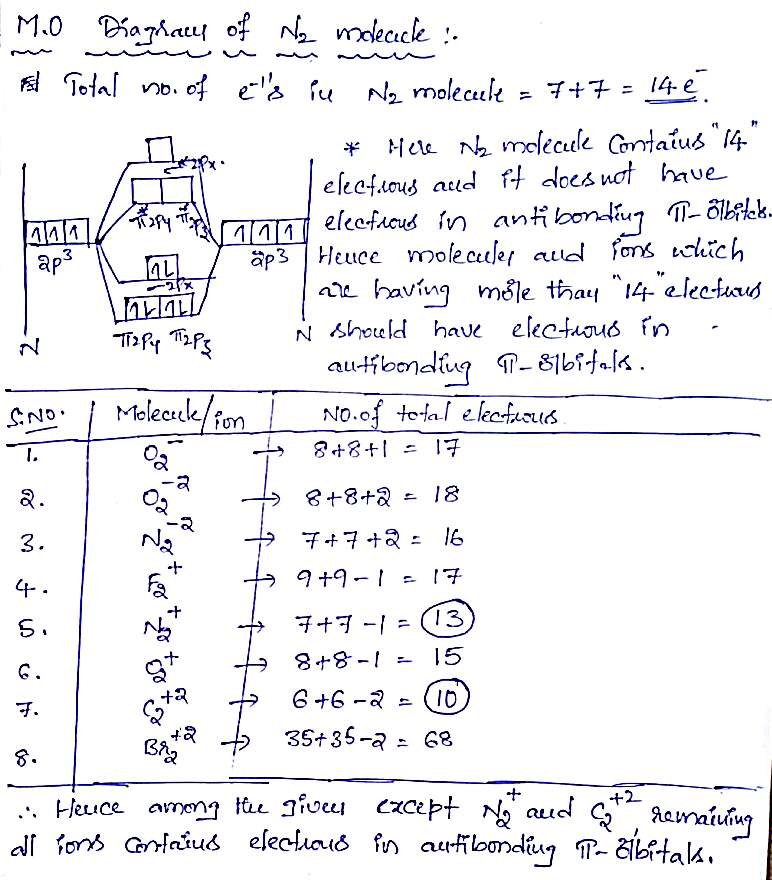
(b) O22-
(c) N22-
(d) F2+
(e) N2+
(f) O2+
(g) C22+
(h) Br22+
Which of the following molecular ions have electrons in π antibonding orbitals?
(a) O2–
(b) O22-
(c) N22-
(d) F2+
(e) N2+
(f) O2+
(g) C22+
(h) Br22+
So much stress and so little time? We’ve got you covered. Get your paper proofread, edited or written from scratch within the tight deadline.


