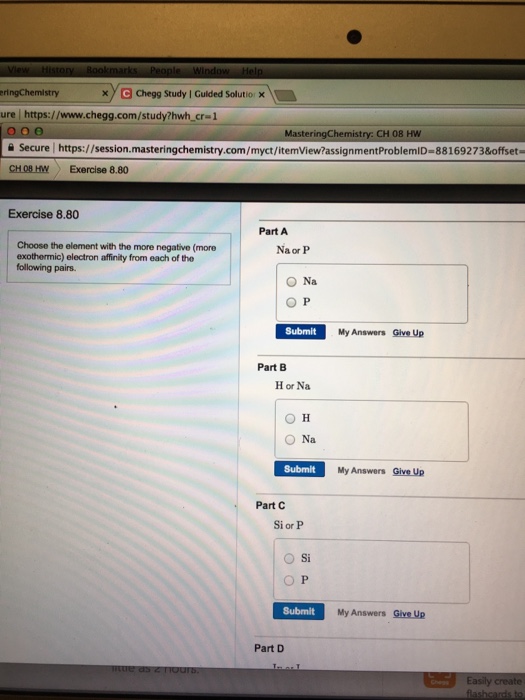
Choose the element with the more negative (more exothermic) electron affinity from each of the following pairs. Na or P Na P H or Na H Na Si or P Si P

Choose the element with the more negative (more exothermic) electron affinity from each of the following pairs. Na or P Na P H or Na H Na Si or P Si P
Part A: Na and P are in same period (3 rd ). As we move from left to right, electron affinity increases.
As Na is in left with respect to P, the electron affinity of Na is less than P.
So, Na has more negative electron affinity.
Part B:
Na has higher electron affinity than H.
So, Na has more negative electron affinity.
Part C: P has more negative electron affinity.
So much stress and so little time? We’ve got you covered. Get your paper proofread, edited or written from scratch within the tight deadline.


