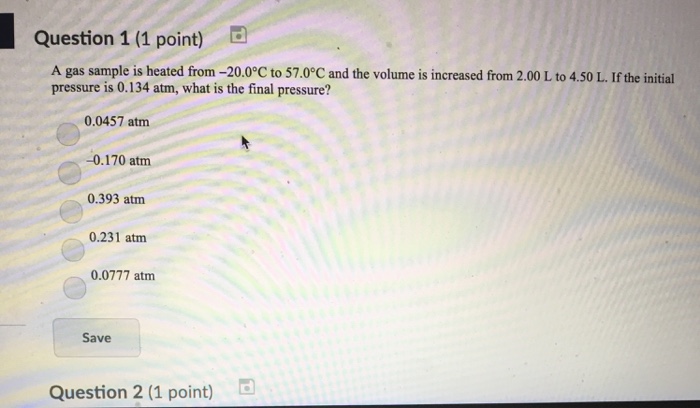
A gas sample is heated from -20.0 Degree C to 57.0 Degree C and the volume is increased from 2.00 L to 4.50 L. If the initial pressure is 0.134 atm, what is the final pressure? 0.0457 atm -0.170 atm 0.393 atm 0.231 atm 0.0777 atm

A gas sample is heated from -20.0 Degree C to 57.0 Degree C and the volume is increased from 2.00 L to 4.50 L. If the initial pressure is 0.134 atm, what is the final pressure? 0.0457 atm -0.170 atm 0.393 atm 0.231 atm 0.0777 atm

So much stress and so little time? We’ve got you covered. Get your paper proofread, edited or written from scratch within the tight deadline.


